Collaboration reduces barriers to rare earth separation (RARE³ KU Leuven in Chemistry World)
[et_pb_section bb_built=”1″ admin_label=”Section” inner_shadow=”on” fullwidth=”on” _builder_version=”3.0.51″ global_module=”5678″][et_pb_fullwidth_menu admin_label=”Fullwidth Menu” global_parent=”5678″ background_color=”#eef4ef” text_orientation=”center” menu_font_size=”16px” menu_id=”2″ background_layout=”light” submenu_direction=”downwards” fullwidth_menu=”on” dropdown_menu_animation=”fade” active_link_color=”#ffffff” dropdown_menu_bg_color=”#39b54a” dropdown_menu_line_color=”#17b52c” dropdown_menu_text_color=”#ffffff” menu_text_color=”#373737″ menu_font=”Source Sans Pro||||” _builder_version=”3.0.51″ background_position=”top_left” background_repeat=”repeat” background_size=”initial” /][/et_pb_section][et_pb_section bb_built=”1″ admin_label=”Section” fullwidth=”on” specialty=”off” _builder_version=”3.0.51″ global_module=”6884″][et_pb_fullwidth_image admin_label=”Fullwidth Image” src=”https://solvomet.eu//wp-content/uploads/2017/06/Solvomet.jpg” show_in_lightbox=”off” url_new_window=”off” use_overlay=”off” border_style=”solid” animation=”off” module_id=”featured-image” _builder_version=”3.0.51″ background_position=”top_left” background_repeat=”repeat” background_size=”initial” /][et_pb_fullwidth_code admin_label=”Fullwidth Code” module_id=”featured-overlay” background_position=”top_left” background_repeat=”repeat” background_size=”initial” /][/et_pb_section][et_pb_section bb_built=”1″ admin_label=”section”][et_pb_row admin_label=”row”][et_pb_column type=”2_3″][et_pb_post_title admin_label=”Post Title” title=”on” meta=”on” author=”off” date=”on” categories=”off” comments=”off” featured_image=”on” featured_placement=”above” parallax_effect=”off” parallax_method=”on” text_orientation=”left” text_color=”dark” text_background=”off” text_bg_color=”rgba(255,255,255,0.9)” use_border_color=”off” border_color=”#ffffff” border_style=”solid” saved_tabs=”all” global_module=”6299″ /][et_pb_text admin_label=”Text”]
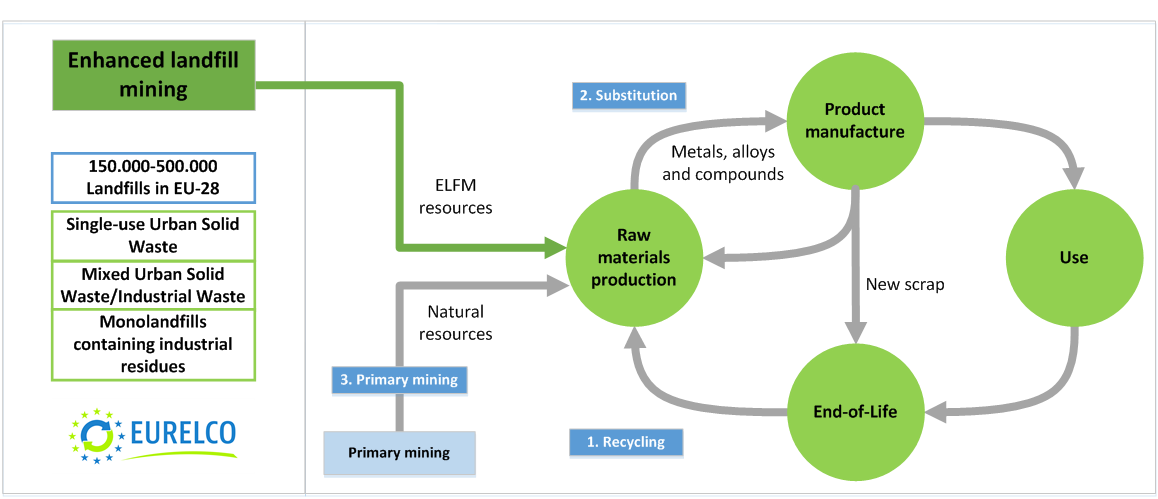
As part of ongoing research into critical metal reuse and recycling, chemical engineers have teamed up with chemists to develop a simple photochemical method for separating vital rare earth metals, europium and yttrium.1Rare earth elements are crucial for modern technologies such as batteries, magnets and consumer electronics, but their similar chemical properties make them difficult to separate. Currently only China performs the extended series of solvent extraction steps needed to produce significant amounts of pure metals from mixed ores. Development of cleaner and less intensive separation methods would remove a barrier to worldwide recycling and production of rare earths.Tom Van Gerven, at the University of Leuven in Belgium, is interested in how light and other alternative energy sources can boost chemical processes. Koen Binnemans, at the same university, is a chemist who has previously applied ionic liquids to rare earth recycling, successfully extracting red lamp phosphor, a mixture of europium and yttrium, from light bulb waste. Together they realised that they could use photochemistry to go a step further and separate europium and yttrium from each other. ‘In the work on the ionic liquid you extract the europium and yttrium in the same ratio as they were in the red lamp phosphor, so you can only use this mixture again for red lamp phosphors. Whereas in our case we are separating the europium and yttrium … so we can use those streams for any purpose we like,’ explains Van Gerven.Both elements exist as trivalent (Eu3+ and Y3+) ions in solution, but europium is also stable in the divalent state (Eu2+). Absorbing light of the correct wavelength reduces Eu3+ to Eu2+, which can then be precipitated out of solution as europium sulfate. For artificial Eu–Y mixtures, the Leuven team removed nearly all of the europium, and almost no yttrium, using a low pressure mercury lamp.
 Photochemical reduction of europium and subsequent precipitation looks to be a promising technique for selectively removing it
Photochemical reduction of europium and subsequent precipitation looks to be a promising technique for selectively removing itThe process also uses isopropanol as a scavenger to remove hydroxyl radicals that oxidise Eu2+ back to Eu3+, instead of the more common but harmful formic acid.
Rare earth element researcher Jean-Claude Bünzli, from the Swiss Federal Institute of Technology, Lausanne, says that this in an improvement on previous work: ‘[they are] revisiting a photo-reduction process proposed in the 1980s by Chinese researchers2 and turning it into a more environmentally friendly process, in particular in avoiding the use of formic acid as scavenger.’
The team also successfully separated a real world industrial sample, albeit with a reduced europium recovery of 50%. They are now working to improve on this, as well as experimenting with alternative low energy light sources such as light emitting diodes. Ultimately, they hope to find methods for photochemically reducing other rare earth metals to process further waste materials or even raw ores.
References
- B Van den Bogaert et al, Green Chem., 2015, DOI: 10.1039/c4gc02140a (This article is free to access until 8 July 2015.)
- R-X Li, X-J Li and R-D Yang, Acta Chimica Sinica, 1989, 47, 861
http://www.rsc.org/chemistryworld/2015/05/rare-earth-separation-recycling-photochemistry
[/et_pb_text][/et_pb_column][et_pb_column type=”1_3″][et_pb_sidebar admin_label=”Sidebar” orientation=”right” area=”sidebar-1″ saved_tabs=”all” remove_border=”off” background_layout=”light” global_module=”5903″ /][/et_pb_column][/et_pb_row][/et_pb_section]