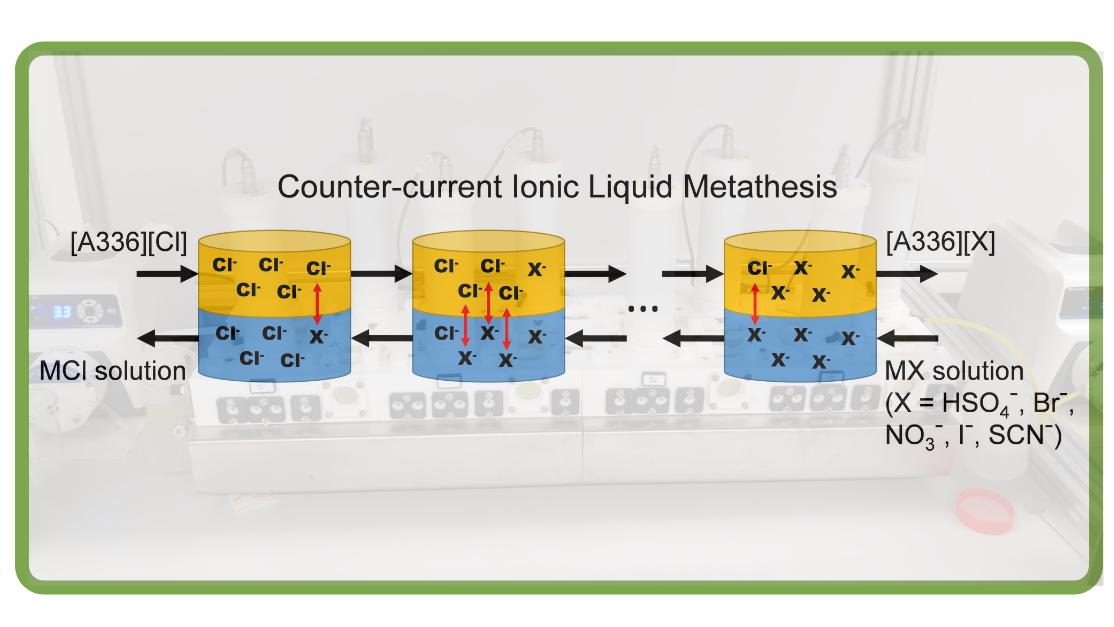
SIM² KU Leuven – SOLVOMET researchers have studied the metathesis or anion-exchange of ionic liquids in a laboratory-scale mixer-settler set-up. The counter-current operation maximises the driving force for the exchange process and essentially allows higher product conversions to be achieved with reduced amounts of reagent. The work was published in ACS Sustainable Chemistry & Engineering.
Ionic liquid synthesis
Ionic liquids (ILs) are solvents solely consisting of ions. Remarkable properties such as a wide liquidus range, intrinsic electrical conductivity, extremely low vapour pressure, and low flammability have made them a promising class of materials and interesting, environmentally-friendly alternatives for volatile molecular solvents. They have been applied as solvents in organic synthesis, extractants for solvent extraction, and solvents for the processing of (bio)polymers.
The majority of ILs is prepared via a two-step synthesis method. The first step constitutes cation formation through the quaternisation of a precursor. This is mostly performed using a haloalkane, resulting in the formation of the corresponding halide salt. In the second step, the targeted anion is introduced via a metathesis or anion-exchange reaction. For hydrophobic ILs, this anion metathesis can easily be performed by contacting the IL with an aqueous solution of the desired anion.
For most purposes where the anion-exchange of a hydrophobic IL is required, this is performed in a multistep batch process where the IL is contacted multiple times with a fresh aqueous salt solution of the desired anion. This means, however, that often a significant excess of the reagent is consumed and wasted because of the relatively high number of contacts required to reach adequate conversions.
Counter-current mixer-settler metathesis
In this work, a counter-current flow diagram was considered for the anion-exchange process. In counter-current mode, the driving force behind the process, i.e. the anion concentration gradient between the two phases, is maximised resulting in the more efficient usage of the reagent. For example, in the first and last contacts of a counter-current process, a fresh IL is contacted with a largely depleted aqueous phase or an almost completely converted IL is contacted with a fresh salt solution.
The metathesis of the IL Aliquat 336, to its bisulfate, bromide, nitrate, iodide, and thiocyanate analogues was studied using a laboratory-scale counter-current mixer-settler setup. Significantly higher IL conversions were achieved, combined with a reduced reagent consumption and waste production, compared with the conventional multistep batch process.
The improvement was most pronounced for the anions placed lowest in the Hofmeister series, e.g. bisulfate, bromide and nitrate, for which the anion exchange is inherently more difficult. Calculation of green metrics such as the reaction mass efficiency (RME) and the environmental factor (E-factor) allowed quantification of this improved anion-exchange efficiency.
The RMEs of the multistep batch experiments were as low as 38% of the values of the counter-current experiments, indicating that a significant improvement in reagent use was achieved in counter-current mode.
Concurrent with this improved reagent use, a reduction in the E-factor, which quantifies waste production, was observed. The countercurrent operation allowed values to be lowered by a factor up to 6.8.
Full reference of paper
Vereycken, W.; Riaño, S.; Van Gerven, T.; Binnemans, K. Continuous Counter-Current Ionic Liquid Metathesis in Mixer-Settlers: Efficiency Analysis and Comparison with Batch Operation. ACS Sustain. Chem. Eng. 2022, 10 (2), 946–955.
https://doi.org/10.1021/acssuschemeng.1c06873.
Acknowledgements
The research was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Program: Grant Agreement 694078 (SOLCRIMET). The authors also thank the KU Leuven for financial support (project C24/18/042, ISOMER).