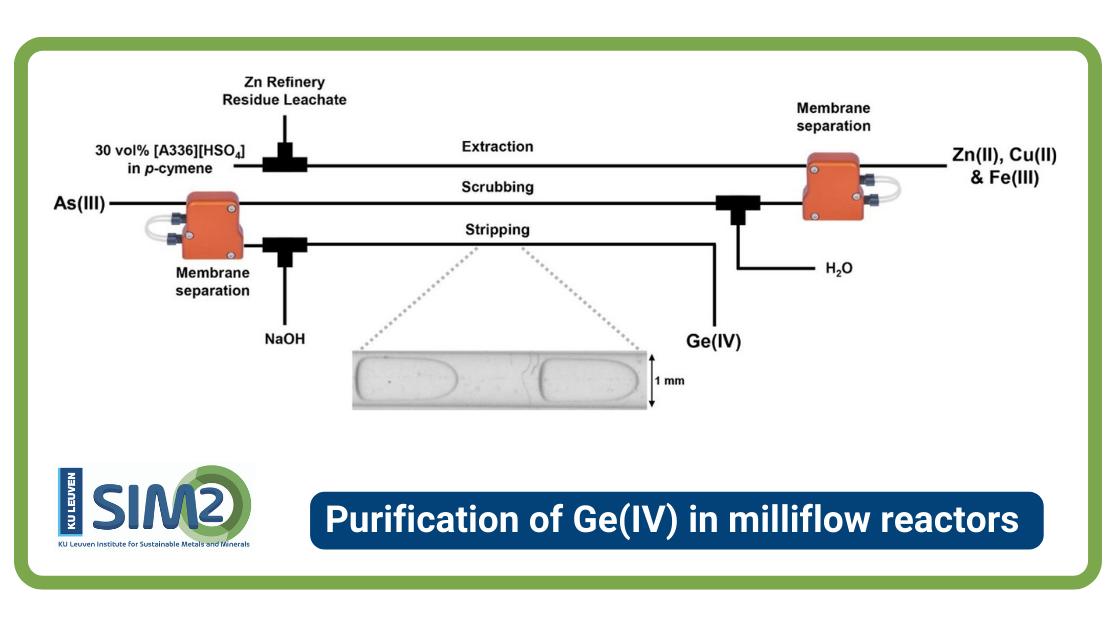
In a fruitful collaboration of SOLVOMET/SIM2 with the group of Prof. Tom Van Gerven (KU Leuven), researchers have demonstrated that the dilution of the ionic liquid [A336][HSO4] improves the hydrodynamics and the mass-transfer performance of the purification of Ge(IV) by solvent extraction in milliflow reactors. Furthermore, the dilution not only allowed to reduce reagent consumption and improved the extraction selectivity but also allowed the use of in-line membrane-based phase separators. The integrated reactor was operated continuously during 4h without any loss in separation performance or stability.
Using in-line membrane-based phase separation
In millifluidic reactors, membrane separators have become popular for the separation of the phases. Usually the continuous phase is permeated over the membrane while the dispersed phase remains in the retentate. Because of the hydrophobic nature of the tubing used in this type of reactors, the organic phases always form the continuous phase and therefore hydrophobic membranes are selected to allow permeation of the continuous organic phase. The required pressure for permeation over the membrane is dependent on the viscosity and can be expected to be relatively high for ionic liquids which can result in failure of the separation module.
One way to lower viscosity is to increase the temperature, however working at elevated temperatures can reduce the life time of the membrane. Another way to lower viscosity is to use diluents. In this work, the aromatic solvent p-cymene was used because it can be considered a greener alternative to toluene as it can be derived from biomass. For the current system, reduction of the viscosity of the organic phase and use of a membrane with a sufficiently small pore size allowed a good separation performance. Fig. 1 shows a schematic representation of the experimental setup used during the single- and multi-stage continuous millifluidic experiments.
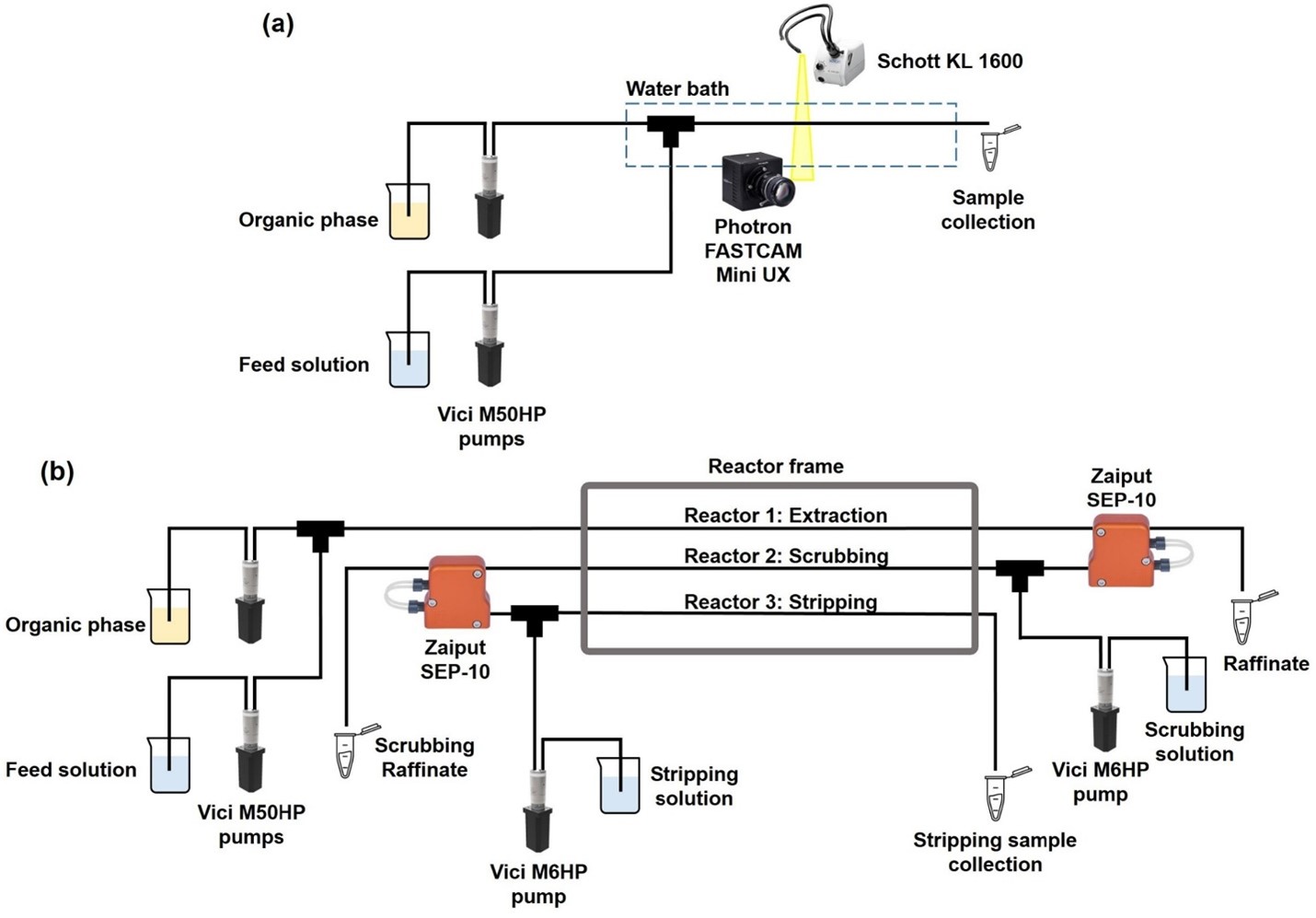
Figure 1. (a) Schematic representation of the single-stage setup. (b) Schematic representation of the multi-stage setup with integrated in-line phase separation.
Integration of extraction, scrubbing and stripping operations
The dilution of [A336][HSO4] improved the hydrodynamics, optimized mass-transfer, increased extraction selectivity and reduced chemicals consumption in the system. The performance of the integrated setup was subsequently evaluated on two different aspects: (1) selectivity, i.e. whether or not the correct metal ions are collected in the correct fractions; and (2) stability during extended operation.
The system displayed the correct selectivity, with Ge(IV) being extracted with some As(III) and small variations on the expected values for the stripping and scrubbing efficiencies. The system also proved to be stable over time, the test was carried out for approximately 33 times the total residence time of the setup. The fact that no significant change in the performance was observed over this time period is testimony to the high stability of the slug flow regime and membrane modules.
In this work we have shown the advantages of diluting ionic liquids when using milliflow devices. The study of both diluted and undiluted [A336][HSO4] ionic liquid systems allowed us to conclude that the dilution of the ionic liquid brings improves the separation in terms of hydrodynamics, mass-transfer, chemical performance and the ability to integrate several millifluidic operations such as extraction, scrubbing and stripping with a membrane-based phase separator.
Full reference paper
Vereyken, W., van Stee, J., Riaño S., Van Gerven, T., Binnemans, K., (2022) “Effect of dilution on the performance of ionic liquids in milliflow solvent extraction applications: Towards integration of extraction, scrubbing and stripping operations with in-line membrane-based phase separation”. SEPARATION AND PURIFICATION TECHNOLOGY, 2022, 297, 121519, doi.org/10.1016/j.seppur.2022.121519
Acknowledgements
The authors thank the KU Leuven for financial support (project C24/18/042, ISOMER). Joren van Stee thanks FWO-Flanders for a SB PhD fellowship (1S47720N).