Within the framework of the Horizon 2020 project CROCODILE, SOLVOMET/SIM² researchers developed a green solvometallurgical cobalt recovery process that combines solvoleaching with solvent extraction. The results have been published in the journal Green Chemistry. The developed process applies the principles of process intensification, thereby decreasing the overall environmental footprint of the overall lithium-ion battery recycling flowsheet.
Primary demand for cobalt set to surge
To achieve climate neutrality by 2050, many industry sectors must undergo a radical transformation. This will see batteries – and lithium-ion batteries (LIBs) in particular – acting as key enablers for decarbonising the energy and mobility sectors. According to the International Energy Agency (IEA) and its report on The Role of Critical Minerals in Clean Energy Transitions, this will see the demand for cobalt (Co) increase 21 times in less than 20 years, as cobalt is used in may different types of cathode materials within lithium‑ion batteries (LIBs).
Recycling can ease the pressure on primary mining
As indicated by the IEA, the amount of spent LIBs from Electric Vehicles reaching the end of their first life is expected to surge after 2030, “at a time when mineral demand is set to still be growing rapidly”. Although recycling of End-of-Life LIBs cannot substitute for the primary mining of energy-transition metals such as cobalt or lithium, it can ease the pressure on primary mining. Developing cost-effective, green recycling methods for spent LIBs is thus of essential importance. One way to limit the environmental impact of LIB recycling processes is to implement the principles of process intensification, such as reducing the number of process steps in the overall flowsheet.
Cobalt recovery from spent LIBs
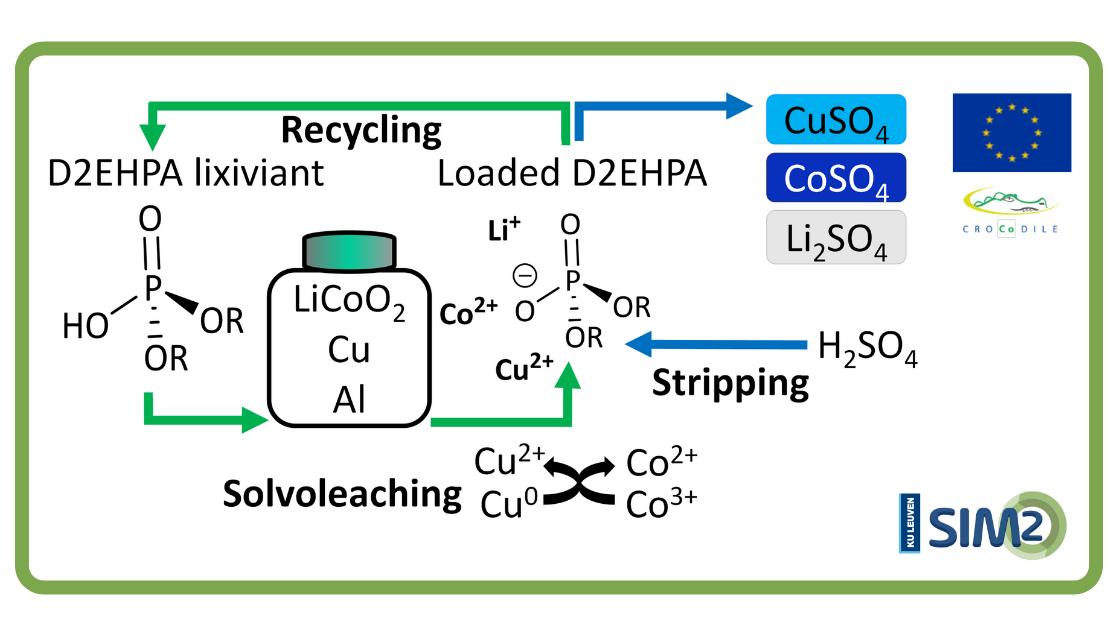
This has been the approach chosen by SOLVOMET/SIM² researchers. They developed a new flowsheet that uses the commercially available extractant D2EHPA to solvoleach cobalt from lithium cobalt oxide (a LIB cathode) in presence of the current collectors aluminium and copper. Cobalt was directly loaded onto the organic D2EHPA phase, assisted by copper that reduced cobalt(III) in lithium cobalt oxide to the more soluble cobalt(II).
Hereafter, cobalt was selectively stripped by sulphuric acid. This approach avoided the pre-separation of the current collectors and reduced the number of stages and consumption of chemicals by directly creating a loaded organic phase, thus omitting the extraction step. Hence, the direct solvoleaching by an extractant presented in this work could potentially intensify cobalt recovery processes. This work could hopefully function as a concept for further implementations in other recovery routes.
Full reference of paper
Peeters, N., Binnemans, K., & Riaño, S. (2022). Recovery of cobalt from lithium-ion battery cathode material by combining solvoleaching and solvent extraction. Green Chemistry. https://doi.org/10.1039/D1GC03776E (gold open access paper).
Acknowledgements
This research project has received funding from the European Union’s EU Framework Programme for Research and Innovation Horizon 2020 under Grant Agreement No 776473 (CROCODILE).