The high thermal stability of Cyphos IL 101 allowed SIM² KU Leuven researchers to investigate the electrochemical behavior of indium at temperatures ranging from 100 °C to 180 °C. The electrochemical deposition of indium followed a two-step process, leading to droplet-like deposits. The work was published in Green Chemistry.
Indium is important for the electronics industry. Indium-containing semiconductors, such as copper indium gallium selenide (CIGS) and indium tin oxide (ITO), are essential for components of many electronic and optoelectronic devices (e.g. detectors, lasers, photovoltaic devices and light-emitting diodes). Indium also finds application in low-temperature alloy solders. Electrochemical deposition is one of many different fabrication techniques for these materials, next to closed-space sublimation, metal organic chemical vapor deposition, co-evaporation and molecular beam epitaxy.
The advantages of electrodeposition over the other techniques are: lower required temperatures, the possibility to work at ambient pressure with a high deposition rate, easily controllable and low-cost equipment and inexpensive precursors. Electrochemical deposition of indium is also of importance for the purification of indium, as high-purity indium is often required as a starting material for the above-mentioned applications. Electrowinning of indium can be an essential step in the recovery of indium from primary and secondary sources.
Electrochemical deposition from ionic liquids
Electrochemical deposition of indium from aqueous electrolytes is often complicated due to the simultaneous formation of hydrogen gas, which decreases the electrodeposition efficiency and can cause dendritic growth, pinholes and hydrogen embrittlement. The use of aprotic ionic liquid electrolytes can circumvent the complications associated with hydrogen evolution. Moreover, ionic liquids exhibit a good electrical conductivity, low vapor pressure, good thermal stability and a wide electrochemical window, allowing deposition of metals that cannot be deposited from water, e.g. aluminum.
The good thermal stability of ionic liquids allows for high-temperature electrodeposition using a liquid cathode. The use of a liquid cathode offers several advantages: (1) easy separation of electrolysis products due to the large density difference between the electrolyte and the liquid cathode, (2) a constant electrode surface area that permits stable values for the process parameters, (3) irregular crystal growth is avoided, so lower operating voltages are required due to the shorter distance between cathode and anode, (4) easier coalescence of microdrops and metallic fog and (5) the possibility to design a continuous process. The use of ionic liquids for electrowinning at temperatures above the melting point of indium can circumvent the complications associated with hydrogen evolution and can offer at the same time the advantages accompanied with the use of a liquid cathode.
Electrochemical deposition of indium from Cyphos IL 101
The electrochemical behavior of indium in the ionic liquid trihexyl(tetradecyl)phosphonium chloride (Cyphos IL 101) was investigated at 120 °C and 180 °C. Commercial Cyphos IL 101 had to be purified due to interference of impurities in the electrochemical measurements. The presence of electroactive impurities in ionic liquids can cause an apparent narrowing of the true electrochemical window. The electrochemical deposition of indium followed a two-step process, involving the reduction of indium(III) to indium(I), followed by the reduction of indium(I) to indium(0).
The indium deposition process has a current efficiency of 70%, leading to the conclusion that part of the current is used for the electrochemical reduction of indium(III) to indium(I) that subsequently undergoes disproportionation to indium(III) and indium(0).
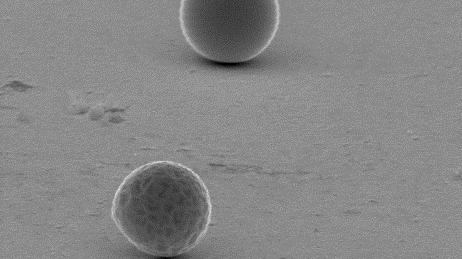
Indium was deposited at temperatures ranging from 100 to 180 °C. The morphology of the indium deposits was examined by scanning electron microscopy. At all temperatures, droplet-like deposits of indium were observed. The formation of droplets has a twofold origin: melting-point depression of very small primary indium particles in combination with undercooling.
The capability of the electrolyte to eliminate iron(III) and zinc(II) electrochemically was also tested by adding indium(III) together with zinc(II) and iron(III) to Cyphos IL 101. Zinc(II) was not co-deposited with indium(III) because the formation of the Lewis-basic ZnCl42− anions shifts the deposition potential outside of the cathodic limit of the phosphonium cation. Iron(III) could not be separated from indium(III) in this way.
The indium speciation in purified Cyphos IL 101 was investigated with Extended X-ray Absorption Fine Structure. The indium speciation is dependent of the molar ratio of indium(III) chloride to the organic chloride salt (commonly expressed as the effective mole fraction of ‘monomeric’ indium(III) chloride, xInCl3). Cyphos IL 101 with a composition of xInCl3 = 0.50 have been shown to contain the [InCl4]− anion as the major indium species. As less indium(III) chloride was added to the mixture, the major indium species gradually changed to [InCl5]2− at xInCl3 = 0.33 and 0.20.
It has to be noticed that the bond distance increased slightly with decreasing amount of indium(III) chloride dissolved in Cyphos IL 101, from 2.430(2) at xInCl3 = 0.33 to 2.440(2) at xInCl3 = 0.20, thus indicating that a small, but significant and increasing, fraction of [InCl6]3− is present when lowering the indium(III) concentration. A diffusion coefficient of (7.5 ± 0.1) × 10−12 m2 s−1 was obtained for the chloroindate(III) anion in Cyphos IL 101 (10 mM In, 120 °C).
Full reference of paper
Clio Deferm, João C. Malaquias, Bieke Onghena, Dipanjan Banerjee, Jan Luyten, Harald Oosterhof, Jan Fransaer and Koen Binnemans, Electrodeposition of indium from the ionic liquid trihexyl(tetradecyl)phosphonium chloride, Green Chem., 2019, 21, 1517-1530 – DOI: 10.1039/C8GC03389G
Acknowledgements
This research was supported by the Flemish Institute for the Promotion of Innovation by Science and Technology (IWT Vlaanderen) via a Baekeland PhD fellowship to Clio Deferm (IWT 130305) and by the Umicore Group Research & Development. The research leading to these results also received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme: Grant Agreement 694078—Solvometallurgy for critical metals (SOLCRIMET).