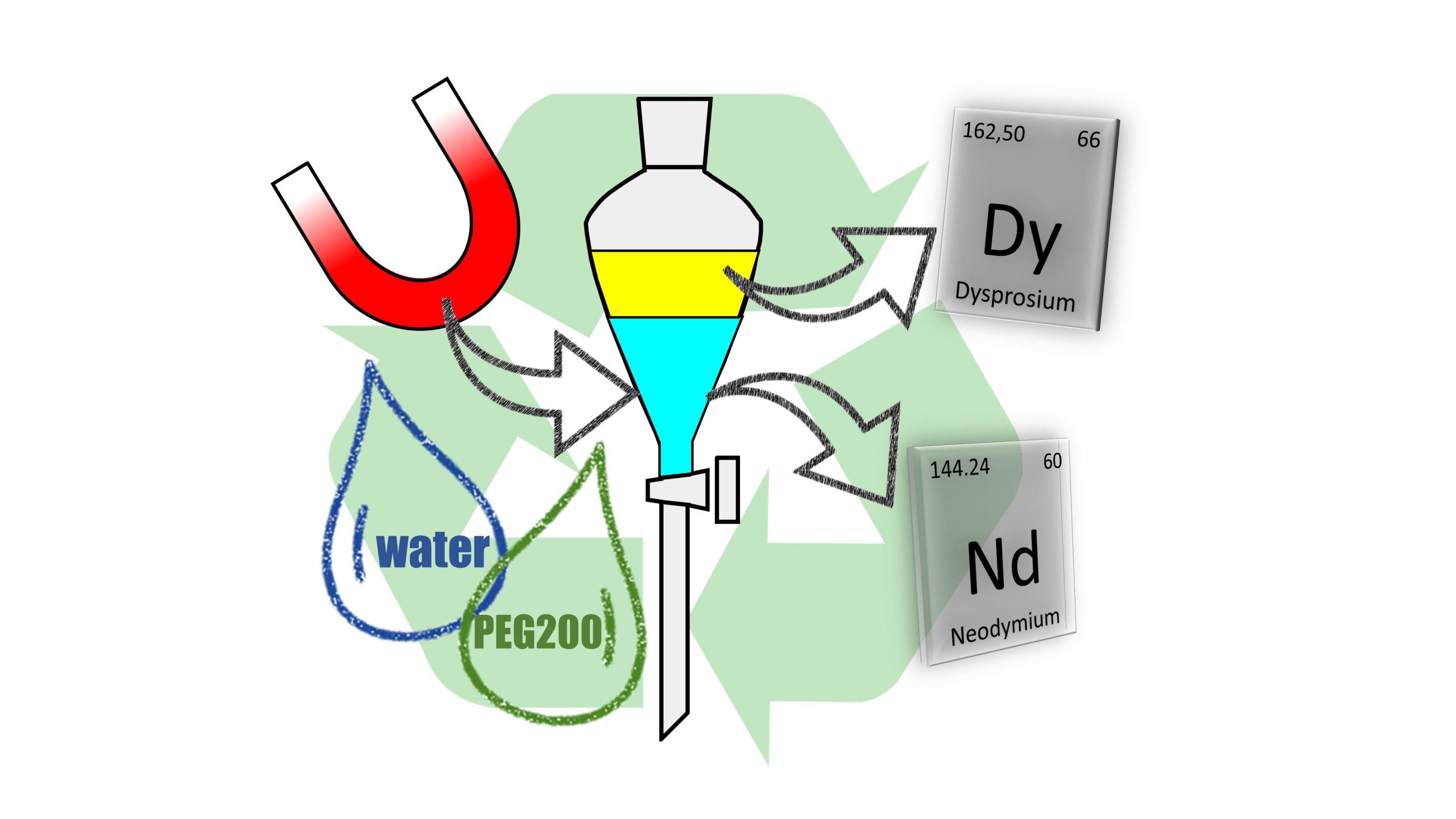
SOLVOMET/SIM² KU Leuven researchers have enhanced the separation of neodymium and dysprosium by replacing the aqueous phase in their solvent extraction process largely by the green solvent poly(ethylene) glycol 200. As water is not completely absent, the solvometallurgical system can be integrated into hydrometallurgical flow sheets by combining it for instance with aqueous leaching of waste magnet material. The work, which was performed in the framework of the ERC SOLCRIMET project, was published in the journal ACS Sustainable Chemistry & Engineering.
As the world is committing itself to become more climate neutral, Nd-Fe-B magnets are having a key role in the current green revolution. The ever-increasing demand for Nd-Fe-B magnets highlights the mounting urgency to recycle the valuable elements in waste magnets.
Non-aqueous solvent extraction can help
While direct magnet-to-magnet recycling is possible in some cases, in others the recovery of the main rare-earth elements, neodymium and dysprosium, is the only feasible route. However, the conventional aqueous solvent extraction commonly used for the separation of these two elements often displays small separation factors, causing recovery difficulties and engineering challenges.
Replacing water by a polar organic solvent, such as the green solvent poly(ethylene) glycol 200 (PEG 200), alters the solvation of rare-earth ions and thus changes the efficiency with which each rare-earth ion is extracted. Separations that are difficult in aqueous environment suddenly become more feasible.
This is exactly what was observed for the separation of a synthetic neodymium/dysprosium mixture, mimicking real magnet leachate, from an HCl solution of PEG 200 30 vol% water and using the neutral extractant Cyanex 923.
A separation factor of 42 was attained, and the resulting counter-current solvent extraction process would only need three stages of extraction and at least one of scrubbing for complete separation of the two rare-earth elements. Moreover, the use of acid for stripping and base for pH control, which is necessary in traditional separation schemes using for instance acidic extractants, is significantly reduced.
Bridging the gap
Solvent extraction as a separation technique is not a stand-alone procedure; there is a downstream process with which it should be integrated, consisting of transforming the waste magnet into a processable material, dissolution of the metals from the solid material, followed by purification.
Efficient hydrometallurgical processes can be used for this, resulting in an aqueous solution of metal salts or a metal precipitate. As up to 30 vol% of water is added to the PEG 200 solution in our new solvent extraction process, it is easy to combine it with these conventional hydrometallurgical processes, simply by adding the aqueous rare-earth salt solutions to pure PEG 200 or by adding aqueous HCl to PEG 200 in order to dissolve solid rare-earth concentrates.
By bridging the gap between hydrometallurgy and solvometallurgy, processes such as rare-earth separation can be easily enhanced.
Full reference paper
Brecht Dewulf, Nagaphani Kumar Batchu, Koen Binnemans, Enhanced Separation of Neodymium and Dysprosium by Nonaqueous Solvent Extraction from a Polyethylene Glycol 200 Phase Using the Neutral Extractant Cyanex 923. ACS Sustain. Chem. Eng. 2020, 51 (8), 19032-19039. https://doi.org/10.1021/acssuschemeng.0c07207.
Acknowledgements
The research leading to these results received funding from the European Research Council (ERC) under the European Union’s Horizon2020 Research and Innovation Programme: Grant Agreement 694078—Solvometallurgy for critical metals (SOLCRIMET). Project website: SOLCRIMET – Advanced ERC Grant